
The innovative hybrid Cobalt-Chromium Abluminal Biodegradable Polymer DES
BioMatrix Alpha™ presents the best in class stent platform design with unique pro-healing coating from the pioneer in abluminal biodegradable technology3.
It combines the proven safety of a DES with an abluminal biodeg radable polymer, the proven efficacy of BA9™ and an innovative cobalt-chromium stent platform design4.
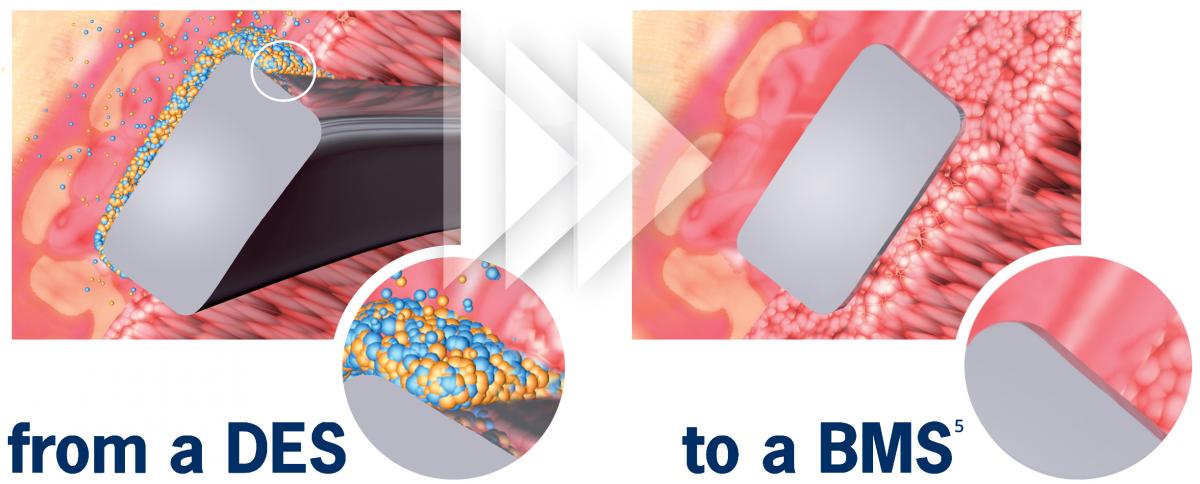
No drug carrier or drug inside the stent
> Early BMS-like endothelial coverage6
> More targeted drug release
> Reduced systemic exposure
BA9™ Designed Specifically for Coronary Stent Application

> Unique drug and proprietary of Biosensors International Group, Ltd.
> Designed for properties that would support healing and re-endothelialization
> Chemical characteristics allow:
- a rapid transfer to the vessel wall, in the absence of a polymer or carrier
- with no loss to the systemic system.
Alpha by Design best in class performance vs other stents9
* Including iterations of the Nobori stent
1. Thin Strut CoCr Biodegradable Polymer Biolimus A9-Eluting Stents versus thicker Strut Stainless Steel Biodegradable Polymer Biolimus A9-Eluting Stents: Two-Year Clinical Outcomes. Menown I et al. Journal of Interventional Cardiology, vol. 2021, Article ID 6654515, 7 pages, 2021
2. Data on file Biosensors International Group, Ltd.
3. Improved safety and reduction in stent thrombosis associated with biodegradable polymer-based biolimus-eluting stents versus durable polymer-based sirolimus-eluting stents in patients with coronary artery disease: final 5-year report of the LEADERS (Limus Eluted From A Durable Versus ERodable Stent Coating) randomized, noninferiority trial. Serruys P. et al JACC Cardiovasc Interv. 2013 Aug;6(8):777-89 First clinical evidence characterizing safety and efficacy of the new CoCr Biolimus-A9 eluting stent: The Biomatrix Alpha™ registry. Menown I et al. Int J Cardiol Heart Vasc. 2020 Jan 27;26:100472
4. Data on file at Biosensors International Group Ltd. Bench test performned on 3.0mm stents
5. In vivo testing in porcine model demonstrates abluminal coating is absorbed after 6 to 9 months. Data on file at Biosensors International
6. Data on file at Biosensors International
7. Biosensors International data on file
8. Sirolimus analog lipophilicity dictates release kinetics and tissue retention after implantation of polymer free drug eluting stents. R. Tzafriri, Poster Presentation EuroPCR 2017
9. Biosensors International internal bench testing performed on 3.0 mm stents. Data on file at Biosensors International
10. Percentage change in stent length after applying 5N compression force longitudinally
11. Recoil measured as percentage change in diameter at RBP



