
The innovative hybrid Cobalt-Chromium Abluminal Biodegradable Polymer DES
BioMatrix™ Alpha presents the best in class stent platform design with unique pro-healing coating from the pioneer in abluminal biodegradable technology3.
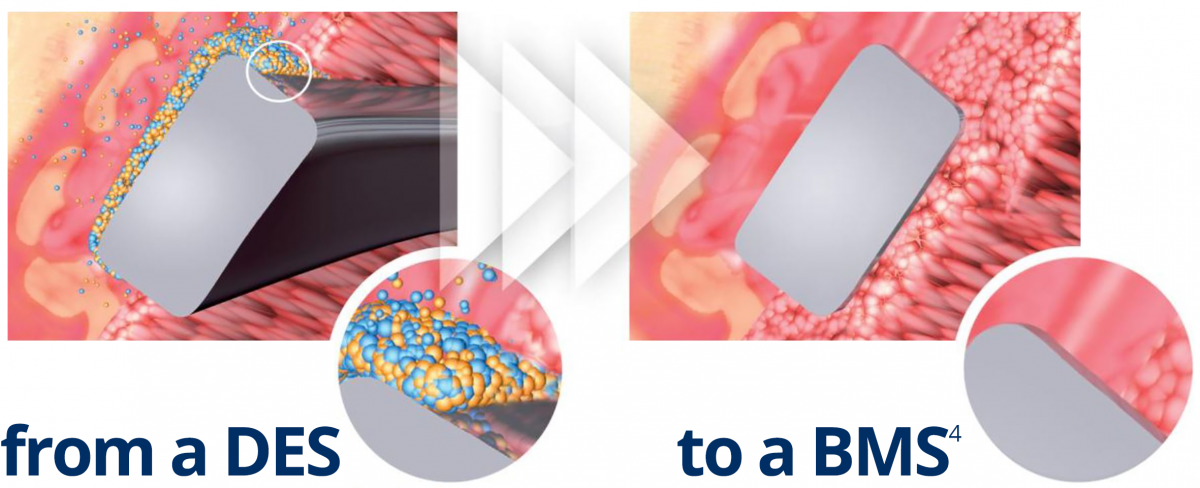
Abluminal coating absorbed after 6 to 9 months4
No drug carrier or drug inside the stent
- Early BMS-like endothelial coverage2
- More targeted drug release
- Reduced systemic exposure
BA9™ Designed Specifically for Coronary Stent Application

- Unique drug and proprietary of Biosensors International Group, Ltd.
- Designed for properties that would support healing and re-endothelialization
- Chemical characteristics allow:
- with no loss to the systemic system.
Alpha by Design best in class performance vs other stents2






