CAUTION: Please note that the following pages are exclusively reserved for Health Care Professionals in countries with applicable health authority product registrations. To the extent this site contains information intended for use by licensed medical professionals, such materials are not intended to offer professional medical advice. Prior to use, please consult device labeling for prescriptive information and operating instructions. Please contact your Biosensors International representative for availability or the products and registration status.
The law restricts these devices to sale by or on the order of a physician. Prior to use, it is important to read the "Instructions for Use" supplied with these devices for indications, contraindications, suggested procedures, warnings, and precautions.
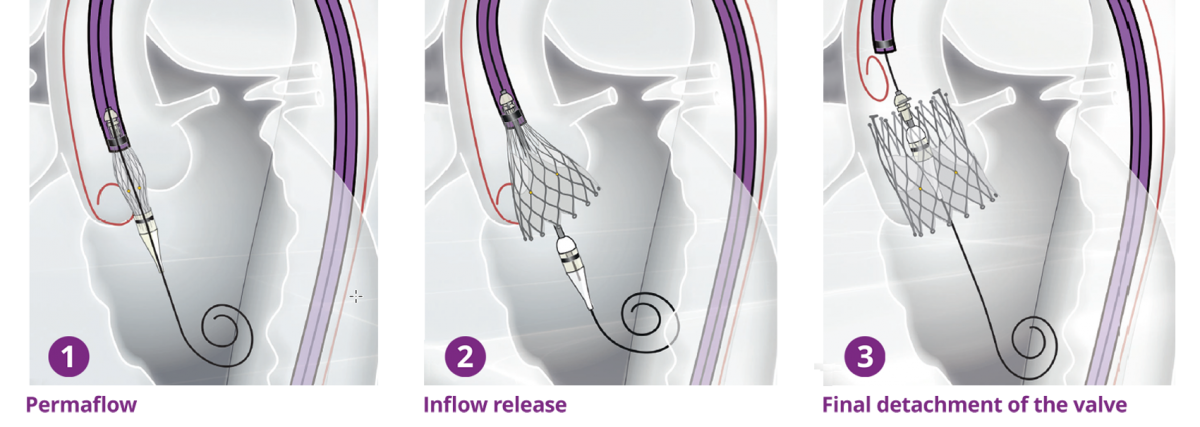
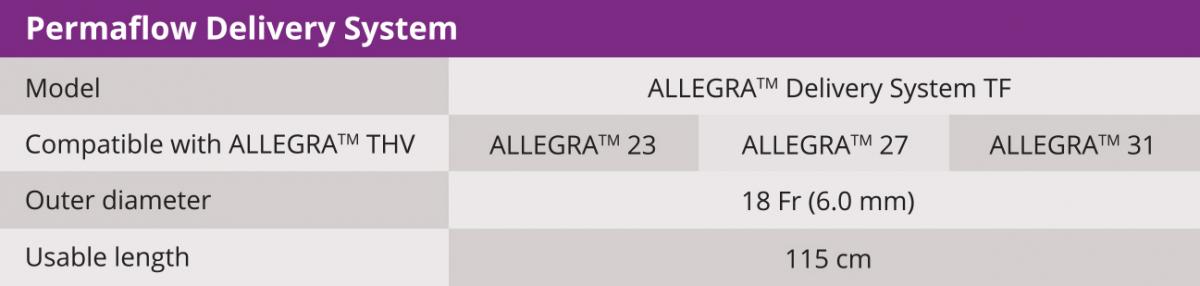
Biosensors’ interventional cardiology products, including BioMatrix™ Alpha, BioFreedom™, BioFreedom™ Ultra, RISE™ NC and RISE™ SC and BioMC™, are not available for sale in the United States and certain other countries. ALLEGRA™ is a product of NVT GmbH. Blue Sail Medical Co., Ltd is the ultimate parent company of NVT GmbH and Biosensors International Group, Ltd. and its subsidiaries are collaborating for the commercialization of the ALLEGRA™ device.
BioMatrix Alpha, BioFreedom, BioFreedom Ultra, Rise NC, Rise SC and BioMC are trademarks or registered trademarks of Biosensors International Group, Ltd. ALLEGRA is a trademark or registered trademark of NVT AG. All other cited trademarks are the property of their respective owners.